
Review unit, Video 1 (Exercise 1.1)
Good morning, everyone, I think we’d better get started. My name is Dr Steven Bishop. Thank you very much for coming along to my presentation today. Now, for the next 10 minutes or so, I’m going to talk about a substance that we’re all very familiar with and one that none of us can do without. Does anyone have any idea of what that might be?
Question from the audience: Are you going to talk about food?
No, it’s not food, but you’re thinking along the right lines. Anyone else, perhaps?
Question from the audience: Is it something that we drink? Is it water?
Yes, of course. What could be more important than water. Basically, I’m going to talk about the chemical substance H2O, better known as water … and to highlight the importance of this substance, the presentation is entitled ‘Water: the amazing liquid of life’.
Now, I’ve divided my presentation into three parts. To start, I’m going to talk about where water comes from and where the earth keeps most of its current reserves. Then, I’d like to focus on what I’ll call ‘the wonders of water’ and give you some interesting facts about its physical properties. And to finish off, I’ll say a little about some key features of our great oceans and seas.
So let’s start then, with where water comes from; its early history, if you like.
Now, the first point I’d like to make is that the Earth retains all of the water ever created – an amount that some scientists have estimated as a massive 326 million cubic miles – so when we’re talking about water, it’s important to remember that we’re referring to a substance that has been used, purified and then reused for about 3,000 million years.
But where did it all come from in the first place? Well, the accepted scientific theory of how water was first created states that when the Earth was formed, a chemical reaction took place between the gases hydrogen and oxygen. As you know, the chemical name for water is H2O which means two parts hydrogen and one part oxygen. So basically, the way it worked is that these gases, hydrogen and oxygen, were up in the atmosphere, circling the newly-formed Earth, and as they reacted together, they became liquid particles and formed a dense layer of cloud. As the temperatures dropped, this layer of cloud released its water in a torrential downpour that lasted for an amazing 60,000 years, filling the lower land basins and forming the oceans and seas as we know them today. And you know, the truly remarkable thing is that the level of the oceans and the amount of water on the Earth has actually remained relatively stable ever since.
Now, if we think about water reserves and where our water is actually based, as you might expect, the bulk of the earth’s water, about 97.2 per cent of it, lies in the great oceans. 2.15 per cent is held in ice caps and glaciers, and the remainder is spread out over an area ranging from three miles below the earth’s surface to seven miles up in the atmosphere. Some of you might think that rivers and streams are a key storage place for the earth’s water, but in fact, these only account for 0.0001 per cent, or about 300 cubic miles. The other surface water totals around 55,000 cubic miles and can be divided up between inland seas and fresh and saltwater lakes.
Apart from its surface water, the earth has a reserve supply of some 200,000 cubic miles of water below the surface. The water in the upper layer, an area known as the aeration zone, clings to soil and rocks and is either absorbed by plants or returns to the air through evaporation. Other water supplies can be found deeper down in what is known as the saturation zone. The water here feeds swamps, rivers, lakes and wells and it can turn up in the most unlikely places. For example, I’m sure you’ll be surprised to hear that the Sahara Desert has an underground reservoir of about 150,000 cubic miles.
Moving back above ground, the atmosphere holds about 3,100 cubic miles of water- enough to cover the entire planet with one inch of rain if it all came down at the same time. In actual fact, this atmospheric water does eventually fall as rain and is replaced by evaporation every 12 days or so. And despite what the nursery rhyme says about the rain in Spain falling mainly on the plain, most of our rainfall actually ends up in the seas and rivers. Only 1/6 of the planet’s total rainfall soaks into the ground.
Now I’d like to move onto the second part of my presentation today, and look at some of water’s wonders. As a substance, water has a number of unique properties, one of the most interesting ones being the fact that when it meets other materials, it forms a skin. You see, water molecules are more strongly attracted to themselves than to other substances and as a result, they squeeze together forming a dense layer. This phenomenon is called surface tension and sometimes, you can actually see it in action. For example, you may have noticed that insects have this almost magical ability to walk on water – well, that all comes down to water’s surface tension.
Another one of water’s strange properties is that it can absorb more heat than almost any other common substance, without experiencing a considerable rise in temperature. For example, when a kettle of water is put on to boil, it is subjected to a temperature that would cause most other substances to either melt or burst into flames. But in the case of water, it just soaks up the heat until a temperature of 100 degrees centigrade is reached. And as we all know, at this point, water boils and we can actually see steam bubbles bursting through its surface tension.
So that’s what happens when water gets hot, but what about when it gets cold? Well, another one of water’s wonders is that it floats when frozen. This is because although water expands at freezing point, it actually becomes less dense. As we all know, in the Arctic regions, huge ice floes cover the surface of lakes and oceans and basically, these act as a protective, insulating blanket. The ice floes prevent further freezing and help life go on underneath the blanket of ice. So you see, the fact that frozen water floats is a very important point for the survival of both us and our planet, because if it didn’t, the ice would gradually build up from all the ocean beds and cover the entire Earth in a solid glacier-like mass. And needless to say, if that happened, life as we currently know it would simply not be able to survive.
This brings us to the final part of my presentation today. And as I promised at the beginning, to finish off, I’d like to say a little about our great oceans and seas.
When you look out over an ocean or sea, if you were actually able to see beneath the waves, you’d find a whole new world of spectacular canyons, great plains and enormous mountain ranges. One such mountain range, the Mid-Atlantic Ridge, runs for about 10,000 miles between Iceland and the Antarctic, which makes it the largest mountain range in the world. And in fact, believe it or not, Iceland, the Ascension Islands and the Azores are all just examples of this underwater mountain range’s highest peaks.
Now as well as great mountain ranges, our oceans also hide some extremely deep holes and trenches. The deepest known trench is the so-called ‘Challenger Deep’, situated off the island of Guam in the Pacific. This trench goes down for an amazing 7 miles and just to give you some idea of how deep that actually is, if we put Everest into it, there would still be about a mile of water above the summit. Surprising, isn’t it?
Now, I said a moment ago that our oceans contain spectacular canyons. The Hudson Canyon, just off the coast of New York, is 150 miles long and 16,500 feet deep. Creatures living at the bottom of this canyon have to be able to cope with tremendous water pressure. And just to give you some idea of what I mean by that, at a depth of 13,000 feet, the water pressure is nearly three tons to the square foot, so if we’re looking at a depth of over 16,000 feet, clearly only highly-specialized organisms are able to survive; most animals would actually find such living conditions intolerable. The last information I’d like to share with you about the oceans and seas is with regard to their currents and tides. As you’ve probably heard, one of the most powerful ocean currents is known as the Gulf Stream. When this current leaves the Caribbean, it’s about 50 miles wide and up to 1,500 feet deep, and it flows across the Atlantic at a speed of about 5 knots, which in terrestrial terms is equal to almost 6 miles per hour. As for tides, well, as every schoolchild knows, the daily rise and fall of the tides is caused by the gravitational pull of the moon and the sun. The effect of each pull causes, in effect, a bulge in the ocean. Our large tides – the Spring tides – occur when the sun and moon are pulling on the same side of the Earth; when the sun and the moon pull at right angles, we get Neap tides, which are the smallest. The largest tides of all though occur in places where the sea runs into bottlenecks, as in the Bay of Fundy on America’s east coast. And this makes for some truly spectacular viewing.
Well, that pretty much brings us to the end of my presentation today. I’ve talked about a very common subject, water, but as I hope to have shown, H2O truly is our planet’s ‘amazing liquid of life’.
Now, if anyone has any questions, I’ll do my best to answer them.

Review unit, Video 2 (Exercise 1.1)
Click here for supplementary interactive exercises
Introduction
Good morning, everyone, I think we’d better get started. My name is Dr Steven Bishop. Thank you very much for coming along to my presentation today. Now, for the next 10 minutes or so, I’m going to talk about a substance that we’re all very familiar with and one that none of us can do without. Does anyone have any idea of what that might be?
Question from the audience: Are you going to talk about food?
No, it’s not food, but you’re thinking along the right lines. Anyone else, perhaps?
Question from the audience: Is it something that we drink? Is it water?
Yes, of course. What could be more important than water. Basically, I’m going to talk about the chemical substance H2O, better known as water … and to highlight the importance of this substance, the presentation is entitled ‘Water – amazing liquid of life’.
Now, I’ve divided my presentation into three parts. To start, I’m going to talk about where water comes from and where the earth keeps most of its current reserves. Then, I’d like to focus on what I’ll call ‘the wonders of water’ and give you some interesting facts about its physical properties. And to finish off, I’ll say a little about some key features of our great oceans and seas.
So let’s start then, with where water comes from; its early history, if you like.

Review unit, Video 3 (Exercise 1.1)
Click here for supplementary interactive exercises
Part 1
Now, the first point I’d like to make is that the Earth retains all of the water ever created – an amount that some scientists have estimated as a massive 326 million cubic miles – so when we’re talking about water, it’s important to remember that we’re referring to a substance that has been used, purified and then reused for about 3,000 million years.
But where did it all come from in the first place? Well, the accepted scientific theory of how water was first created states that when the Earth was formed, a chemical reaction took place between the gases hydrogen and oxygen. As you know, the chemical name for water is H2O which means two parts hydrogen and one part oxygen. So basically, the way it worked is that these gases, hydrogen and oxygen, were up in the atmosphere, circling the newly-formed Earth, and as they reacted together, they became liquid particles and formed a dense layer of cloud. As the temperatures dropped, this layer of cloud released its water in a torrential downpour that lasted for an amazing 60,000 years, filling the lower land basins and forming the oceans and seas as we know them today. And you know, the truly remarkable thing is that the level of the oceans and the amount of water on the Earth has actually remained relatively stable ever since.
Now, if we think about water reserves and where our water is actually based, as you might expect, the bulk of the earth’s water, about 97.2 per cent of it, lies in the great oceans. 2.15 per cent is held in ice caps and glaciers, and the remainder is spread out over an area ranging from three miles below the earth’s surface to seven miles up in the atmosphere. Some of you might think that rivers and streams are a key storage place for the earth’s water, but in fact, these only account for 0.0001 per cent, or about 300 cubic miles. The other surface water totals around 55,000 cubic miles and can be divided up between inland seas and fresh and saltwater lakes.
Apart from its surface water, the earth has a reserve supply of some 200,000 cubic miles of water below the surface. The water in the upper layer, an area known as the aeration zone, clings to soil and rocks and is either absorbed by plants or returns to the air through evaporation. Other water supplies can be found deeper down in what is known as the saturation zone. The water here feeds swamps, rivers, lakes and wells and it can turn up in the most unlikely places. For example, I’m sure you’ll be surprised to hear that the Sahara Desert has an underground reservoir of about 150,000 cubic miles.
Moving back above ground, the atmosphere holds about 3,100 cubic miles of water- enough to cover the entire planet with one inch of rain if it all came down at the same time. In actual fact, this atmospheric water does eventually fall as rain and is replaced by evaporation every 12 days or so. And despite what the nursery rhyme says about the rain in Spain falling mainly on the plain, most of our rainfall actually ends up in the seas and rivers. Only 1/6 of the planet’s total rainfall soaks into the ground.

Review unit, Video 4 (Exercise 1.1)
Click here for supplementary interactive exercises
Part 2
Now I’d like to move onto the second part of my presentation today, and look at some of water’s wonders. As a substance, water has a number of unique properties, one of the most interesting ones being the fact that when it meets other materials, it forms a skin. You see, water molecules are more strongly attracted to themselves than to other substances and as a result, they squeeze together forming a dense layer. This phenomenon is called surface tension and sometimes, you can actually see it in action. For example, you may have noticed that insects have this almost magical ability to walk on water – well, that all comes down to water’s surface tension.
Another one of water’s strange properties is that it can absorb more heat than almost any other common substance, without experiencing a considerable rise in temperature. For example, when a kettle of water is put on to boil, it is subjected to a temperature that would cause most other substances to either melt or burst into flames. But in the case of water, it just soaks up the heat until a temperature of 100 degrees centigrade is reached. And as we all know, at this point, water boils and we can actually see steam bubbles bursting through its surface tension.
So that’s what happens when water gets hot, but what about when it gets cold? Well, another one of water’s wonders is that it floats when frozen. This is because although water expands at freezing point, it actually becomes less dense. As we all know, in the Arctic regions, huge ice floes cover the surface of lakes and oceans and basically, these act as a protective, insulating blanket. The ice floes prevent further freezing and help life go on underneath the blanket of ice. So you see, the fact that frozen water floats is a very important point for the survival of both us and our planet, because if it didn’t, the ice would gradually build up from all the ocean beds and cover the entire Earth in a solid glacier-like mass. And needless to say, if that happened, life as we currently know it would simply not be able to survive.

Review unit, Video 5 (Exercise 1.1)
Click here for supplementary interactive exercises
Part 3
This brings us to the final part of my presentation today. And as I promised at the beginning, to finish off, I’d like to say a little about our great oceans and seas.
When you look out over an ocean or sea, if you were actually able to see beneath the waves, you’d find a whole new world of spectacular canyons, great plains and enormous mountain ranges. One such mountain range, the Mid-Atlantic Ridge, runs for about 10,000 miles between Iceland and the Antarctic, which makes it the largest mountain range in the world. And in fact, believe it or not, Iceland, the Ascension Islands and the Azores are all just examples of this underwater mountain range’s highest peaks.
Now as well as great mountain ranges, our oceans also hide some extremely deep holes and trenches. The deepest known trench is the so-called ‘Challenger Deep’, situated off the island of Guam in the Pacific. This trench goes down for an amazing 7 miles and just to give you some idea of how deep that actually is, if we put Everest into it, there would still be about a mile of water above the summit. Surprising, isn’t it?
Now, I said a moment ago that our oceans contain spectacular canyons. The Hudson Canyon, just off the coast of New York, is 150 miles long and 16,500 feet deep. Creatures living at the bottom of this canyon have to be able to cope with tremendous water pressure. And just to give you some idea of what I mean by that, at a depth of 13,000 feet, the water pressure is nearly three tons to the square foot, so if we’re looking at a depth of over 16,000 feet, clearly only highly-specialized organisms are able to survive; most animals would actually find such living conditions intolerable. The last information I’d like to share with you about the oceans and seas is with regard to their currents and tides. As you’ve probably heard, one of the most powerful ocean currents is known as the Gulf Stream. When this current leaves the Caribbean, it’s about 50 miles wide and up to 1,500 feet deep, and it flows across the Atlantic at a speed of about 5 knots, which in terrestrial terms is equal to almost 6 miles per hour. As for tides, well, as every schoolchild knows, the daily rise and fall of the tides is caused by the gravitational pull of the moon and the sun. The effect of each pull causes, in effect, a bulge in the ocean. Our large tides – the Spring tides – occur when the sun and moon are pulling on the same side of the Earth; when the sun and the moon pull at right angles, we get Neap tides, which are the smallest. The largest tides of all though occur in places where the sea runs into bottlenecks, as in the Bay of Fundy on America’s east coast. And this makes for some truly spectacular viewing.
Well, that pretty much brings us to the end of my presentation today. I’ve talked about a very common subject, water, but as I hope to have shown, H2O truly is our planet’s ‘amazing liquid of life’.
Now, if anyone has any questions, I’ll do my best to answer them.

Review unit, Video 6 (Exercise 1.4)
Click here for supplementary interactive exercises
Well, that pretty much brings us to the end of my presentation today. I’ve talked about a very common subject, water, but as I hope to have shown, water truly is our planet’s ‘amazing liquid of life’. Now, if anyone has any questions, I’ll do my best to answer them.
Question 1: I have a question. In your presentation, you said that the Earth retains all of the water ever created. Do you mean to say that no new water is ever produced?
Answer: That’s a good question … (fade out)
Question 2: In your presentation, you said that the oceans were created by a downpour that lasted for 60,000 years. How exactly do we know that?
Answer: OK, let me explain. For one thing … (fade out)
Question 3: I’m still not sure about how surface tension works. Can you go over that part again?
Answer: Sorry if that wasn’t very clear. You know what I was saying about insects … (fade out)
Review unit, Video 7 (Exercise 1.4)
Well, that pretty much brings us to the end of my presentation today. I’ve talked about a very common subject, water, but as I hope to have shown, water truly is our planet’s ‘amazing liquid of life’. Now, if anyone has any questions, I’ll do my best to answer them.
Question 1: I have a question. In your presentation, you said that the Earth retains all of the water ever created. Do you mean to say that no new water is ever produced?
Answer: That’s a good question …

Review unit, Video 8 (Exercise 1.4)
Question 2: In your presentation, you said that the oceans were created by a downpour that lasted for 60,000 years. How exactly do we know that?
Answer: OK, let me explain. For one thing …

Review unit, Video 9 (Exercise 1.4)
Question 3: I’m still not sure about how surface tension works. Can you go over that part again?
Answer: Sorry if that wasn’t very clear. You know what I was saying about insects …

Review unit, Video 10 (Exercise 2.6)
Click here for supplementary interactive exercises
a) So let’s start then, with where water comes from; its early history, if you like.
b) Now, the first point I’d like to make is that the Earth retains all of the water ever created.
c) The accepted scientific theory of how water was first created states that when the Earth was formed, a chemical reaction took place between the gases hydrogen and oxygen.
d) As the temperatures dropped, this layer of cloud released its water in a torrential downpour that lasted for an amazing 60,000 years.
e) The truly remarkable thing is that the level of the oceans and the amount of water on the Earth has actually remained relatively stable ever since.

Review unit, Video 11 (Exercise 2.7)
Click here for supplementary interactive exercises
f) The Earth’s surface water is stored in rivers, lakes, oceans and seas.
g) Water floats when it’s frozen, doesn’t it?
h) Of course, water floats when it’s frozen, doesn’t it? That’s why we have icebergs.
i) This bring us to the final part of my presentation.
j) Does anyone have any questions?

Review unit, Video 12 (Exercise 2.9)
Click here for supplementary interactive exercises
a) Water in the saturation zone feeds swamps, rivers, lakes and wells.
b) When water boils, we can see bubbles bursting through the surface tension, can’t we? That’s the point when we know its temperature has reached 100°.
c) Are the ice floes really that important?
d) The largest tides occur in places where the sea runs into bottlenecks.

Review unit, Video 13 (Exercise 2.10)
Click here for supplementary interactive exercises
a) The truly remarkable thing is that the level of the oceans has actually remained relatively stable ever since.
b) Most animals would find such living conditions absolutely intolerable.
c) If the ice built up from the ocean beds, life as we currently know it would be completely unable to survive.
d) Without surface tension, it would be totally impossible for insects to walk on water.

Review unit, Video 14 (Exercise 2.11)
Click here for supplementary interactive exercises
e) There can be no doubt whatsoever that water is a very very important substance.
f) There are many many cubic miles of water hidden beneath the Earth’s surface.
g) It is far far easier for us to waste water than it is to save it.
h) As you go deeper, the water pressure becomes greater and greater.
i) Once you get past a few thousand feet, it becomes harder and harder for organisms to survive unless they’re highly specialized.
Review unit, Video 15 (Exercise 3.5)
Click here for supplementary interactive exercises
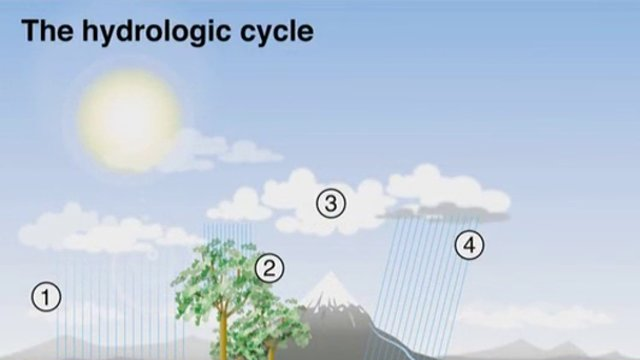
As this diagram shows, the hydrologic cycle, commonly known as the ‘water cycle’ consists of seven major processes. As you can see down here on the left, the first two processes happen at around the same time as each other and are known as EVAPORATION and TRANSPIRATION. Basically, evaporation refers to the process when surface water is converted into water vapour, whereas transpiration refers to the evaporation of water from plants. The third process is called CONDENSATION – this happens when the rising water vapour cools and is converted back into liquid water in the form of clouds. These clouds are then transported across the sky by the wind and eventually, this leads to the fourth main process in the cycle, PRECIPITATION, which refers to the cloud water falling back to Earth in the form of rain, sleet, hail or snow. Once it’s back on the Earth, the water moves into process five, INFILTRATION, which refers to the water draining back into the surface soil. The water then works its way down through these upper layers of soil, a process known as PERCOLATION to join the groundwater already stored under the surface. This then leads to the final stage of the cycle, a process known as RUNOFF. Runoff refers to the movement of ground and surface water back to a main body of water, such as rivers, lakes or seas from which the whole cycle begins again.
